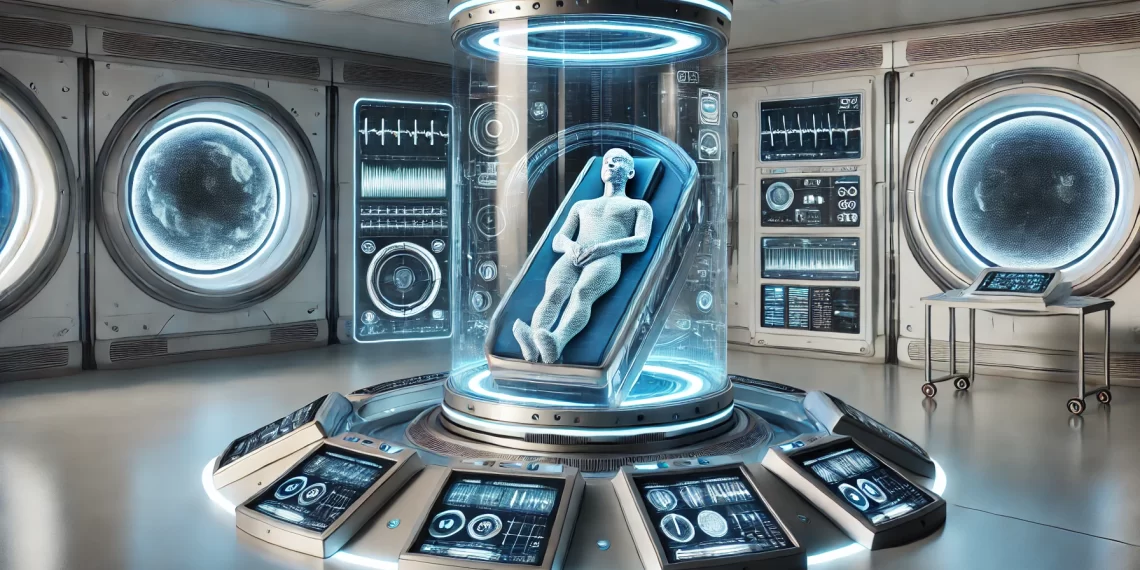
Researchers at Georgia State University have made a groundbreaking discovery in neuroscience, identifying a group of neurons in mice that can be activated to induce a torpor-like state. This state, resembling hibernation, dramatically reduces body temperature, heart rate, and energy expenditure without triggering anxiety or stress behaviors. The implications of this discovery span from revolutionary health treatments to transformative advancements in space exploration and military applications.
The study, published in Nature Metabolism, was led by neuroscience professor Eric Krause and his team, who collaborated with experts from the University of Florida and the Monell Chemical Senses Center. “We found that repeated [chemogenetic] excitation of the neurons decreased body mass and produced a hypometabolic state without inducing anxiety-like behaviors that are often observed with chronic stress,” Krause explained. “This is changing what we know about body-to-brain communication and how profoundly it affects physiology and behavior.”
The neurons, located near the base of the skull, play a critical role in relaying sensations from the gut and heart to the brain. When activated, these neurons simulate the physical sensations of fullness and increased blood pressure, suppressing appetite and significantly lowering metabolic rates. This discovery could be key to addressing pressing health issues like obesity, hypertension, and cardiometabolic diseases.
Beyond its health implications, the ability to induce a controlled hypometabolic state has exciting applications in space travel. Long-duration missions, such as those to Mars or deep-space exploration, could benefit from astronauts entering a hibernation-like state to conserve resources and reduce physiological strain. This natural energy-saving mechanism, already present in some mammals, could revolutionize space exploration by enabling human survival in extreme environments over extended periods.
The military could also find this discovery invaluable. Soldiers deployed in harsh or remote environments could enter a controlled state of torpor to reduce the physical demands of their missions. For example, hypometabolic states might enable prolonged endurance during long-term reconnaissance operations or allow military personnel to survive extended periods without adequate supplies. Additionally, the ability to induce torpor could help medical teams stabilize soldiers with severe injuries by slowing metabolism and preserving vital functions until more advanced care is available.
The study also explored the involvement of oxytocin, the “love hormone,” in regulating these neurons. Researchers found that oxytocin could influence internal sensations, such as “gut feelings,” further broadening the potential applications of this discovery. Guillaume de Lartigue from the Monell Chemical Senses Center noted, “We’ve tapped into the body’s own energy-saving toolkit. By activating these neurons, we can trigger an ancient survival mechanism present in mammals.”
With a $3.4 million grant from the National Institutes of Health, the research team aims to expand its studies, exploring how this hypometabolic state could be applied to humans. This funding underscores the transformative potential of the findings, especially as researchers investigate how body-to-brain communication might mitigate chronic stress and its impact on cardiometabolic health.
The implications for future warfare are especially compelling. Hypometabolic states could allow soldiers and uncrewed systems to operate more efficiently in extreme conditions, such as deep-sea exploration, high-altitude operations, or nuclear-affected regions. Furthermore, the concept of controlled torpor could redefine the logistics of global military strategy, enabling rapid deployment of personnel and systems over long distances with minimal energy requirements.